Which Statement Best Describes the Properties of Ionic Compounds
A It is difficult to synthesise the compounds. Low melting point tendency to transfer electrons b.

Physical Properties Of Ionic Compounds Chemistry For Non Majors
They generally break into pieces when pressure is applied hence they are considered brittle.
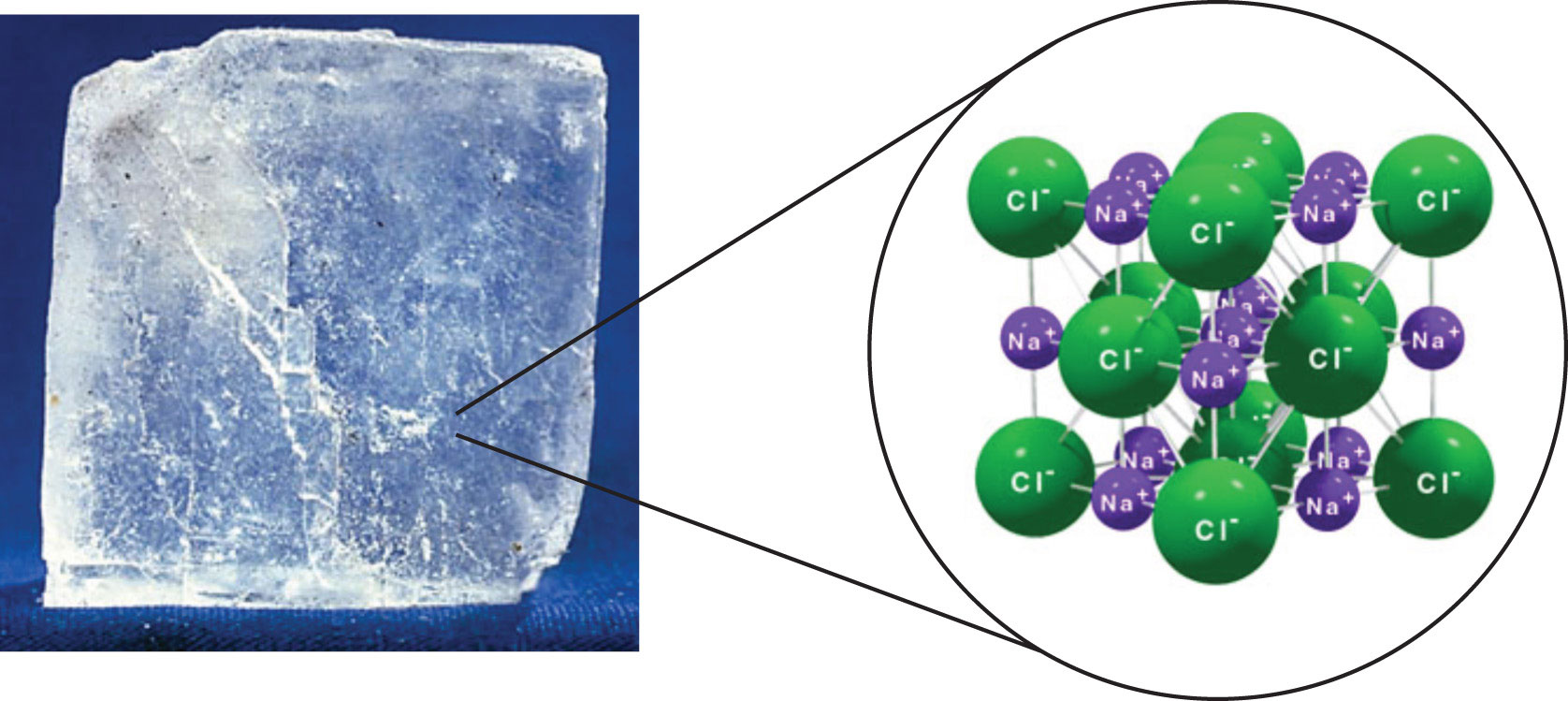
. Ionic compounds are solid and hard because of the strong force of attraction between the positive and negative ions. Volatile c Conduct electricity in the molten state or in an aqueous solution but do not conduct electricity. Ionic compounds are composed of a metal and a nonmetal while covalent compounds are composed of all nonmetals.
C Active compounds are likely to have poor pharmacokinetic properties. They mostly have low melting and boiling points. Covalent compounds Ionic compounds composed of simple molecules a Have high melting and boiling points a Have low melting and boiling points b Exist as solids at room temperature.
They can be oxidized to form carbon dioxide OB. The following properties are all characteristics of ionic compounds EXCEPT. B There is a lack of suitable reagents.
The best property for ionic bonds is its conductivity of electricity when molten. They are made up of particles that are arranged in a repeating pattern. Non-volatile b Usually exist as liquids or gases at room temperature.
High melting and boiling points. An experiment that can be recalled on this is the NaCl in water that is used to light up a bulb. Which statement below best describes what happens when a crystalline ionic compound like NaCl dissolves in water.
They are brittle solid at room temperature and have a high melting point. D Active compounds are likely to have poor. Ionic Compound Properties 1.
There is not a statement available so it is difficult to answer this. The Na and Cl- ions chemically bond with the water molecules forming new ionic compounds that remain in solution. Chemistry questions and answers.
They are always composed of an equal proportion of cations to anions. Ionic compounds have high melting points and boiling points because due to strong force of between the positive and negative charged ions. Which statement is true about a polyatomic ion.
Given the three statements below pick the best answer. Ionic compounds have very low melting points. Which statement is true about ionic compounds.
The statement that best describes the formation of an ionic compound is. Which statement is true about ionic compounds. B D A C Which of the answer choices best describes the structure and properties of.
Ionic compounds are normally in which physical state at room temperature. DMolecular compounds contain highly directional covalent bonds which results in the formation of molecules-discrete particles that do not covalently bond to each other. They are good conductors of heat and electricity and are ductile and malleable.
Some properties of ionic compounds are high melting points solid in room temperature and they are brittle. Which statement best describes how to predict the formula of a stable ionic compound. Physical properties of ionic compounds.
Ionic compounds are highly soluble in water when dissociates. Due to the presence of the strong force of attraction between the positive and negative ions ionic compounds are solids and are hard to break. IThe main forces that make ionic solids stable are the crystal lattice forcesIIThe main force holding covalent molecules together is the sharing of electrons between atomsIIIThe total number of valence electrons for CCl4 is 32.
Which statement best describes the properties of ionic compounds. Which of these compounds is most likely to be ionic. They also have high boiling points because of the ions integrated.
Which of the following is not a characteristic of most ionic compounds. Ionic compounds are very easy to bend. Ionic compounds have higher melting points than covalent compounds.
1 pts Question 1 Which statement best describes what happens when ionic compounds dissolve in water. Their water solubility depends on the relative proportion of polar and nonpolar groups OD. If solid they tend to have low melting points.
They dissociate into ions in aqueous solution O C. High melting point tendency to transfer electrons d. Which of the following best describes the properties of an ionic compound.
Conduct electricity when dissolved in water. Which statement does not correctly describe the properties of most organic compounds. Chemistry questions and answers.
What disadvantage is there in using linear peptide scaffolds in chemical libraries aimed at finding lead compounds. Ions separate from each other and become surrounded by water molecules water molecules surround whole ionic crystals Ocation-anion pairs separate and become surrounded by water molecules O ions separate from each other and keep away from. Melting and boiling points.
Properties Of Ionic Compounds Chemistry Quiz Quizizz Gk Quiz On Ionic Compounds Solved 1 Pts Question 1 Which Statement Best Describes What Chegg Com. High melting point tendency to. Properties of ionic compounds.
Water molecules surround and pull away individual Na and Cl. Melting and Boiling point. Ionic compounds contain ionic bonds in which one atom donates an electron to the other resulting in a new force that holds the ions together in pairs in the solid phase.
Solid ionic compounds are good conductors of electricity. It is made of atoms that are covalently bonded together. Prominent properties of ionic compounds are.
Given the statements what is the correct order of events for the formation of an ionic compound. Low melting point tendency to share electrons c. Ionic compounds have varying solubilities in water.

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Welcome To Learnapchemistry Com Ap Chemistry Chemical Science Ap Chem

Ionic Bond Definition Properties Examples Facts Britannica

What Are Ionic Compounds Definition Structure Properties Examples With Videos Of Ionic Compounds Ionic Character

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

What Are Ionic Compounds Definition Structure Properties Examples With Videos Of Ionic Compounds Ionic Character

Physical Properties Of Ionic Compounds Chemistry For Non Majors

What Are The Three Properties Of Ionic Compounds Quora

Ionic Bond Definition Properties Examples Facts Britannica

Which Of The Following Phrases Best Describes Process Focus In 2022 Process The Selection Focus

Molecules And Compounds Overview Atomic Structure Article Khan Academy

What Are The Three Properties Of Ionic Compounds Quora

Lattice Energy I Ionic Compounds A Basic I Introduction I Lattice Energy Of Ionic Compounds A Basic Youtube Ionic Compound Ionic Bonding Ionic

Lesson Explainer Ionic Bonding Nagwa

Lesson Explainer Ionic Bonding Nagwa
Ch105 Chapter 4 The Shape And Characteristics Of Compounds Chemistry

Welcome To Learnapchemistry Com Ionic Bonding Ap Chem Ap Chemistry
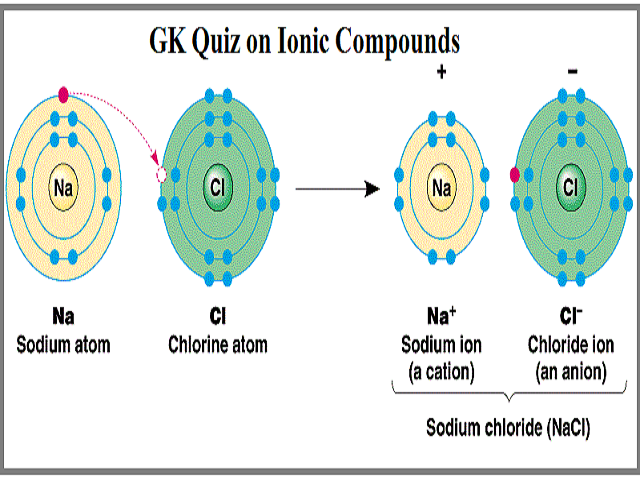
Comments
Post a Comment